Every year, hundreds of thousands of people in the U.S. end up in the hospital because of unexpected side effects from medications they took exactly as prescribed. These aren’t mistakes in dosing or drug interactions from mixing pills - they’re adverse drug reactions (ADRs) caused by how your body naturally processes drugs based on your genes. For many, it’s a matter of bad luck. But what if you could know ahead of time whether a drug is likely to hurt you - not just help you?
Why Some People Have Bad Reactions to Common Medications
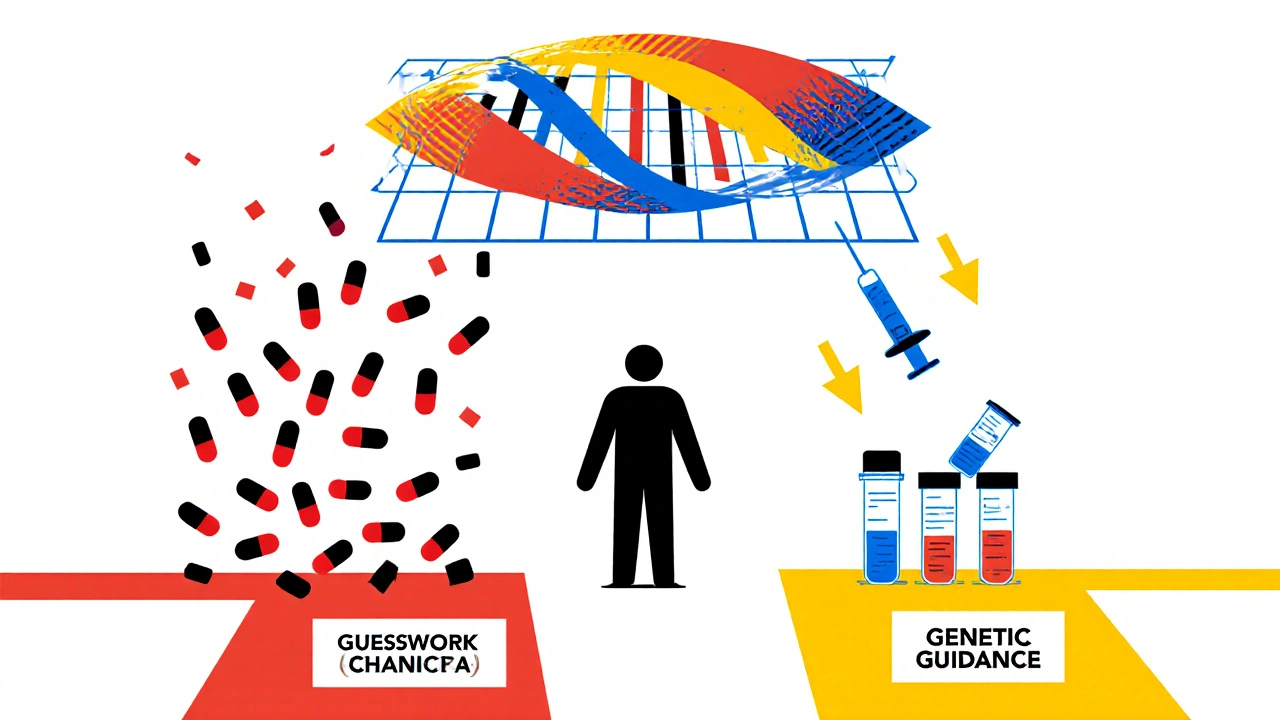
You might think a drug works the same way for everyone. It doesn’t. Two people taking the same dose of the same pill can have wildly different outcomes. One feels better. The other ends up in the ER with a severe rash, liver damage, or dangerously low blood cell counts. Why? Because of genes. Your DNA controls how your liver breaks down drugs, how your body absorbs them, and even how your immune system reacts to them. For example, if you carry the HLA-B*1502 gene variant, taking carbamazepine (a common seizure and nerve pain drug) could trigger Stevens-Johnson syndrome - a life-threatening skin reaction. This isn’t rare in people of Asian descent; up to 15% carry this variant. Without testing, you won’t know until it’s too late. The same goes for warfarin, a blood thinner. Too much can cause internal bleeding. Too little won’t prevent clots. Your CYP2C9 and VKORC1 genes determine how sensitive you are to it. A standard dose might be perfect for one person and deadly for another. Without genetic insight, doctors are guessing.How Pharmacogenetic Testing Works
Pharmacogenetic testing looks at specific genes that affect how your body handles medications. It’s not about predicting disease risk - it’s about predicting drug response. The test is simple: a cheek swab or blood sample is analyzed for variants in key genes like CYP2D6, CYP2C19, TPMT, SLCO1B1, and HLA-B. These genes influence over 100 commonly prescribed drugs, including antidepressants, painkillers, statins, chemotherapy agents, and antiplatelet drugs. The results are grouped into metabolic profiles: poor, intermediate, normal, rapid, or ultrarapid metabolizer. A poor metabolizer of CYP2D6, for instance, can’t break down codeine into its active form - so it doesn’t relieve pain. But if they take tramadol, they might build up toxic levels because their body converts it too slowly. That’s not a dosing error - it’s a genetic mismatch. In 2023, the landmark PREPARE study tested this on nearly 7,000 patients across seven European countries. Before prescribing any new medication, doctors checked their patients’ genetic profiles. The result? A 30% drop in serious adverse reactions compared to standard care. That’s not a small improvement - it’s a game-changer.What Drugs Are Most Affected by Genetic Testing?
Some drugs have well-established gene links. Here are the most critical ones:- Carbamazepine - HLA-B*1502 testing prevents deadly skin reactions in high-risk populations.
- Azathioprine - TPMT testing cuts the risk of bone marrow failure by 78%.
- Clopidogrel - CYP2C19 testing identifies people who won’t benefit from this heart drug - a major cause of repeat heart attacks.
- Statins (like simvastatin) - SLCO1B1 testing predicts muscle damage risk, helping avoid debilitating myopathy.
- Fluoxetine and other SSRIs - CYP2D6 and CYP2C19 variants determine if antidepressants will work or cause side effects like nausea, insomnia, or agitation.
- Codeine and tramadol - Ultrarapid metabolizers convert these to dangerous levels of morphine, risking respiratory arrest - especially in children.

Why Preemptive Testing Beats Reactive Testing
Most doctors still wait until a patient has a bad reaction before considering genetics. That’s like checking your brakes after the car crashes. Preemptive testing - done before any drug is prescribed - is the only way to truly prevent harm. The PREPARE study showed preemptive testing reduced ADRs by 30%. Reactive testing - done after a reaction - only cut them by 15-20%. Why? Because once you’ve had a severe reaction, the damage is done. You might be hospitalized, lose work time, or need emergency treatment. Preventing it is cheaper, safer, and less traumatic. Therapeutic drug monitoring - checking blood levels after you’ve taken a drug - helps adjust doses but doesn’t tell you why the drug behaved that way. Pharmacogenetic testing explains the root cause. It’s not just monitoring - it’s predicting.Cost, Coverage, and Real-World Impact
A full pharmacogenetic panel costs between $200 and $500 in the U.S. That sounds expensive - until you compare it to the cost of an ADR. The average hospital stay for a serious drug reaction runs $15,000 to $40,000. The NHS estimates ADRs cost the UK £500 million a year in avoidable admissions. Pharmacogenetic testing pays for itself. Medicare and Medicaid cover testing for specific high-risk pairs: CYP2C19 before clopidogrel, TPMT before azathioprine, and DPYD before fluorouracil (a chemo drug). Many private insurers are following. A 2021 review of 59 studies found 78% of pharmacogenetic programs were cost-effective - meaning they saved money while improving outcomes. The University of Florida’s personalized medicine program, running since 2012, saw a 75% drop in ADR-related ER visits among tested patients. Their initial $1.2 million investment paid for itself in 18 months.Barriers to Widespread Use
Despite the evidence, adoption is slow. Only 18% of primary care clinics use pharmacogenetic testing. Why? First, many doctors don’t know how to interpret the results. A 2022 survey found only 37% of physicians felt confident reading pharmacogenetic reports. That’s changing - CPIC (Clinical Pharmacogenetics Implementation Consortium) now offers free, updated guidelines for 34 gene-drug pairs, with quarterly updates. Second, integrating results into electronic health records is tricky. If a doctor doesn’t see a genetic alert when prescribing, the test is useless. Systems that auto-flag high-risk combinations - like blocking codeine for ultrarapid metabolizers - work best. Third, diversity matters. Most genetic data comes from people of European descent. Variants common in African, Indigenous, or Asian populations were underrepresented - until recently. In 2024, the NIH added 126 new gene-drug links from underrepresented groups. This is critical. A test that doesn’t work for everyone isn’t truly personalized.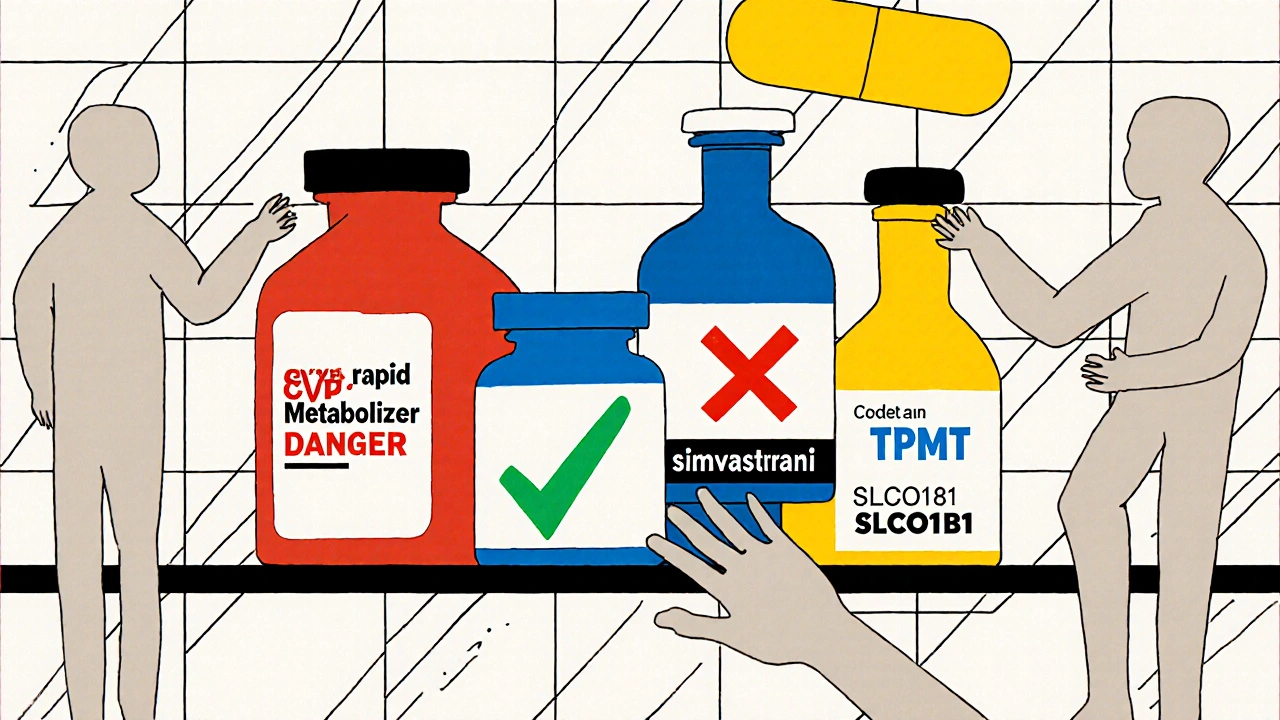
What Patients Say
Patients overwhelmingly support testing. In surveys, 85% say they’d get tested if their doctor recommended it. They want to avoid side effects. They want to know why a drug didn’t work. They want control. But concerns remain. About one-third worry about genetic privacy. Will insurers use this data against them? The Genetic Information Nondiscrimination Act (GINA) protects against health insurance and employment discrimination in the U.S. - but not life insurance or long-term care. That gap still causes anxiety.The Future Is Already Here
The global pharmacogenomics market is set to hit $22.4 billion by 2028. The European Commission is investing €150 million to roll out preemptive testing nationwide by 2027. Major U.S. academic hospitals plan to implement it by 2026. New tools are coming. Point-of-care tests using PCR chips could bring the cost down to $50-$100 by 2026. Polygenic risk scores - combining dozens of gene variants - may soon predict response to complex drugs like antidepressants with 60% greater accuracy than single-gene tests. This isn’t science fiction. It’s medicine evolving. We no longer have to guess what drug will work for you. We can know.What You Can Do Today
If you’ve had a bad reaction to a medication - or if multiple drugs have failed you - ask your doctor about pharmacogenetic testing. It’s not a cure-all, but it’s the most powerful tool we have to prevent harm before it happens. If you’re on long-term meds - especially antidepressants, blood thinners, painkillers, or statins - your genes might be telling you something your doctor hasn’t heard yet. Don’t wait for a reaction. Ask. Get tested. Save yourself from unnecessary risk.What is pharmacogenetic testing?
Pharmacogenetic testing analyzes your DNA to see how your body processes medications. It looks at specific genes that affect drug metabolism, absorption, and immune response to predict whether a drug will work well for you or cause harmful side effects.
Which drugs are most affected by genetic testing?
Drugs with strong gene links include carbamazepine (HLA-B*1502), clopidogrel (CYP2C19), azathioprine (TPMT), simvastatin (SLCO1B1), codeine and tramadol (CYP2D6), warfarin (CYP2C9/VKORC1), and many antidepressants. Over 100 commonly prescribed drugs have known genetic interactions.
Is pharmacogenetic testing covered by insurance?
Yes, for certain high-risk drug-gene pairs. Medicare and Medicaid cover testing for CYP2C19 before clopidogrel, TPMT before azathioprine, and DPYD before fluorouracil. Many private insurers also cover it, especially if there’s a history of adverse reactions or failed treatments.
How accurate is pharmacogenetic testing?
Modern genotyping arrays detect clinically relevant variants with 99.9% accuracy. The results are not probabilistic guesses - they’re direct readings of your DNA. Interpretation depends on clinical guidelines (like CPIC), which are updated quarterly based on new evidence.
Can pharmacogenetic testing help with mental health medications?
Yes. Studies show up to 70% of patients with depression have gene variants affecting how they metabolize SSRIs and SNRIs. Genotype-guided prescribing reduces side effects like nausea, insomnia, and agitation by 40-50% and improves response rates within three months.
Is pharmacogenetic testing worth it if I’m healthy?
Yes. Nearly 94% of people have at least one gene variant that affects drug response. Even if you’re healthy now, you’ll likely need medication at some point. Having your profile on file means future prescriptions can be safer from day one - no trial and error, no ER visits.
Are there risks to getting tested?
The physical risk is minimal - it’s just a cheek swab or blood draw. The main concerns are privacy and psychological impact. GINA protects against health insurance and job discrimination, but not life or long-term care insurance. Some people feel anxious about results, but most report relief knowing why a drug didn’t work or caused side effects.
Comments (10)
Laurie Sala
November 22, 2025 AT 04:45
This is the most important thing I’ve read all year!! I’ve been on 7 different antidepressants-each one made me feel like I was being slowly poisoned-and no one ever asked if my genes might be to blame!! I mean, seriously?? Why are we still playing Russian roulette with prescriptions?? I cried reading this-finally, someone gets it!!
Matthew Mahar
November 23, 2025 AT 05:15
so i just got tested last month bc my doc said my body just 'reacts weird' to meds and turns out im a ultra rapid cyp2d6 metabolizer?? like... i was on codeine for a back injury and it made me feel like i was on heroin?? turns out my body was turning it into morphine like a factory?? now they gave me tramadol and i feel normal?? mind blown. this should be standard. like, why isnt this at every pharmacy??
Lisa Detanna
November 24, 2025 AT 14:34
I work in a rural clinic in Nebraska and we just started offering pharmacogenetic testing six months ago. We’ve had 37 patients tested so far. One woman had been on five different statins over five years-each one gave her muscle pain so bad she couldn’t walk. Her SLCO1B1 test showed she was at extreme risk. We switched her to pravastatin-no side effects. She cried. We all cried. This isn’t futuristic medicine. It’s basic human care. Why are we still waiting for someone to almost die before we listen?
John Mackaill
November 26, 2025 AT 13:53
There’s a quiet revolution happening here. We’ve been doing this at our hospital in Manchester since 2021. Our ER visits for drug reactions dropped 40%. The real win? Patients feel heard. They stop saying, 'I’m just bad with meds' and start saying, 'My body just works differently.' That shift-from blame to biology-is everything. We’re not just preventing harm-we’re restoring dignity.
Adrian Rios
November 27, 2025 AT 13:46
Let me just say this: I’m a 52-year-old guy who’s been on warfarin for atrial fibrillation for 12 years. I’ve had three scary bleeds, two hospitalizations, and I’ve had my INR checked so many times I could probably do it blindfolded. Then last year, they finally did the CYP2C9 and VKORC1 test. Turns out I’m a super slow metabolizer. My dose was triple what I needed. They cut it in half. No more bleeding. No more weekly blood draws. I feel like I’ve been given back my life. And this? This is the kind of thing that should be covered by every insurance plan, every single day, no questions asked. We’re not talking about luxury medicine here-we’re talking about basic science being ignored because it’s inconvenient. It’s criminal.
Casper van Hoof
November 28, 2025 AT 10:32
The epistemological implications of pharmacogenetic testing are profound. It represents a paradigmatic shift from population-based medicine to individuated therapeutic epistemology. The traditional Hippocratic model, predicated on empirical observation and statistical averages, is being supplanted by a genomic hermeneutic that renders the patient not as a statistical outlier but as a unique biological text. This is not merely clinical innovation-it is the ontological reconfiguration of medical authority.
Richard Wöhrl
November 29, 2025 AT 18:45
Just wanted to add a quick note-don’t forget the TPMT test before azathioprine! My cousin was on it for Crohn’s and got full-blown bone marrow failure. They didn’t test. She nearly died. Now, every single person I know with autoimmune issues gets tested before any immunosuppressant. It’s literally a 5-minute swab that saves lives. And the cost? $250. Versus a $50,000 ICU stay? No contest. Also, if you’re on clopidogrel and had a stent, get the CYP2C19 test. If you’re a poor metabolizer, that drug is basically useless-and you’re at risk for a second heart attack. Please, please, please ask your doctor. It’s not scary. It’s life-saving.
Pramod Kumar
December 1, 2025 AT 18:31
Bro, this is the future, man. In India, we’re starting to see this in big hospitals now. My uncle got tested after he had a bad reaction to a cholesterol pill-he got muscle cramps so bad he couldn’t climb stairs. Turned out he had the SLCO1B1 variant. Switched to rosuvastatin, no more pain. He’s hiking now. People say, 'Oh, it’s too expensive,' but I say, 'What’s more expensive? A lifetime of pain or one test?' We’re not just treating disease anymore-we’re decoding bodies. It’s like having a secret manual for your own machine. And guess what? Your genes don’t lie. They’ve been whispering this whole time. We just finally learned how to listen.
Brandy Walley
December 3, 2025 AT 07:00
This is just Big Pharma’s way to sell more tests.
Demi-Louise Brown
December 4, 2025 AT 07:32
As a healthcare provider, I’ve witnessed the transformation this testing brings-not just in outcomes, but in patient confidence. When someone understands why a medication failed them, they stop feeling broken. They stop doubting themselves. They stop believing they’re 'difficult' or 'noncompliant.' This isn’t just science-it’s healing. And while cost and access remain challenges, the data is unequivocal: preemptive testing saves lives, reduces suffering, and cuts long-term costs. We owe it to every patient to offer this. Not as an option. As a standard.