Personalized Cancer Recurrence Risk Assessment
Based on current medical evidence, this tool helps you understand your individual risk when considering immunosuppressant therapy after cancer. All recommendations align with the latest studies published in Gastroenterology and clinical guidelines from major medical societies.
Enter your information above to see personalized recommendations.
Your Assessment Results
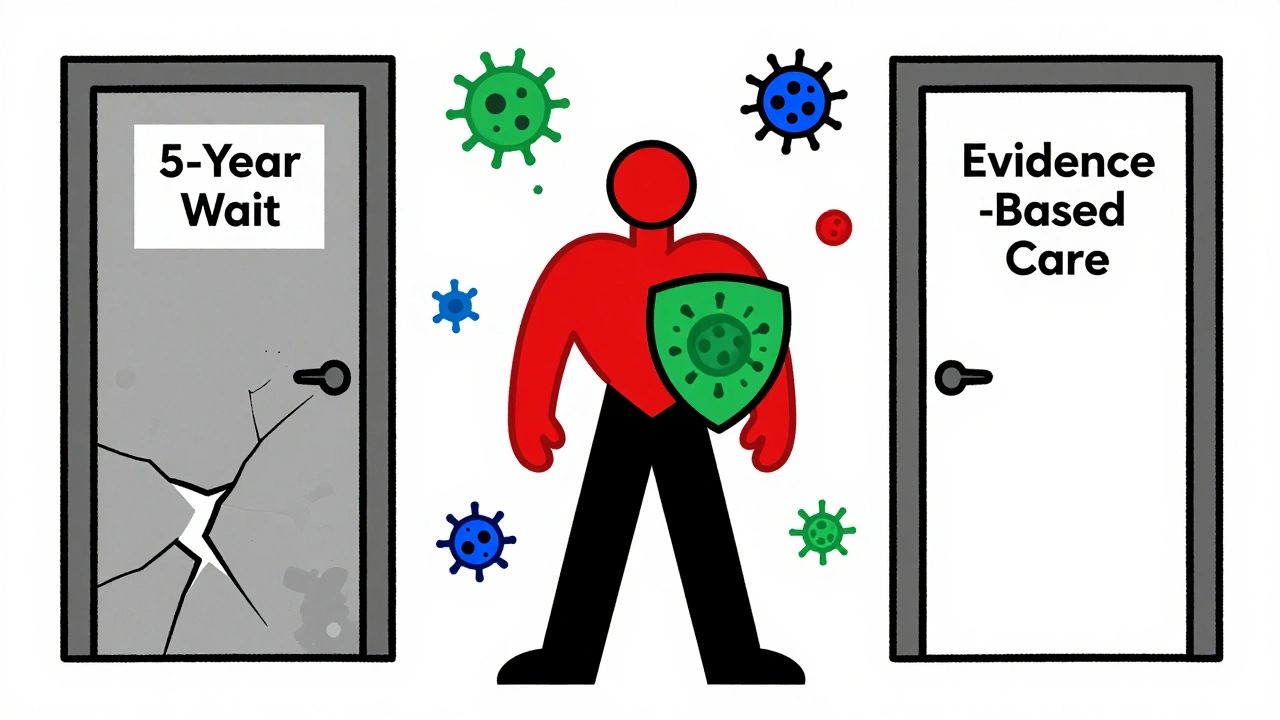
For years, doctors told patients with a history of cancer who needed immunosuppressants to wait at least five years before starting treatment. The fear was simple: if your immune system is turned down, it might not catch cancer coming back. But that advice? It wasn’t based on solid proof. It was guesswork. Now, we have real data-and it changes everything.
There’s No Evidence That Immunosuppressants Cause Cancer to Return
A massive study published in Gastroenterology in 2016 looked at over 11,700 people with autoimmune diseases like rheumatoid arthritis, Crohn’s disease, or psoriasis who had survived cancer. They compared those who took no immunosuppressants with those on anti-TNF drugs like infliximab or adalimumab, traditional drugs like methotrexate or azathioprine, or combinations of all three. The results? No meaningful difference in cancer recurrence rates. The group on combination therapy had the highest number of recurrences-but even that wasn’t statistically significant. In plain terms: immunosuppressants didn’t make cancer come back more often.
That study wasn’t the end. In 2024, researchers doubled the sample size to over 24,000 patients and tracked them for nearly 86,000 person-years. Same result. Anti-TNF agents, JAK inhibitors like tofacitinib, biologics like ustekinumab and vedolizumab-all showed no increased risk. Even patients who started immunosuppressants just six months after cancer treatment had no higher chance of recurrence than those who waited five years. The old rule? Outdated.
Timing Doesn’t Matter-But Cancer Type Still Does
One of the biggest myths was that you had to wait five years before restarting immunosuppressants. That number came from nowhere. No clinical trial ever proved it. The 2024 study found no difference in recurrence whether treatment began before or after the five-year mark. P-value? 0.43. Meaning: the timing didn’t matter.
But here’s the catch: not all cancers are the same. While most types-including breast, lung, colon, and prostate cancer-showed no increased risk with immunosuppressants, melanoma and certain blood cancers like leukemia or lymphoma are different. These cancers rely more heavily on immune surveillance. Experts still advise caution here. If you had melanoma in the last two years, or an active blood cancer, your doctor may hold off longer. But even then, it’s not a blanket ban. It’s a risk-benefit conversation.
What About Newer Drugs? Are They Safer?
It’s not just the old drugs like methotrexate anymore. Today, we have biologics and targeted therapies that are more precise. The 2024 study found that newer agents like ustekinumab and vedolizumab didn’t raise recurrence risk-and in fact, their recurrence rates were slightly lower than those of traditional immunomodulators. Not enough to call it a win statistically, but it’s a trend worth watching.
And here’s something surprising: combination therapy (like taking an anti-TNF with methotrexate) had the highest numerical recurrence rate-54.5 cases per 1,000 person-years. But again, it wasn’t significantly higher than the other groups. So while doctors might avoid combinations in high-risk cases, the data doesn’t prove they’re dangerous. It just means we need to be smarter about who gets them.

What Changed in Clinical Practice?
Before 2016, many rheumatologists and gastroenterologists would refuse to treat patients with a cancer history. Some stopped medications cold. Others delayed treatment for years, even if the patient’s arthritis or IBD was flaring, causing joint damage, hospitalizations, or loss of work. The fear of cancer outweighed the real damage of uncontrolled inflammation.
Now? That’s changing fast. The American College of Rheumatology, the European League Against Rheumatism, and the FDA have all updated their guidance. The FDA revised labeling for multiple immunosuppressants in 2022 to say: “Clinical studies have not shown an increased risk of cancer recurrence in patients with prior malignancy treated with [this agent].”
Prescriptions for biologics in patients with cancer histories jumped 18.7% between 2017 and 2022, according to IQVIA data. That’s not because more people got cancer-it’s because doctors finally felt safe prescribing.
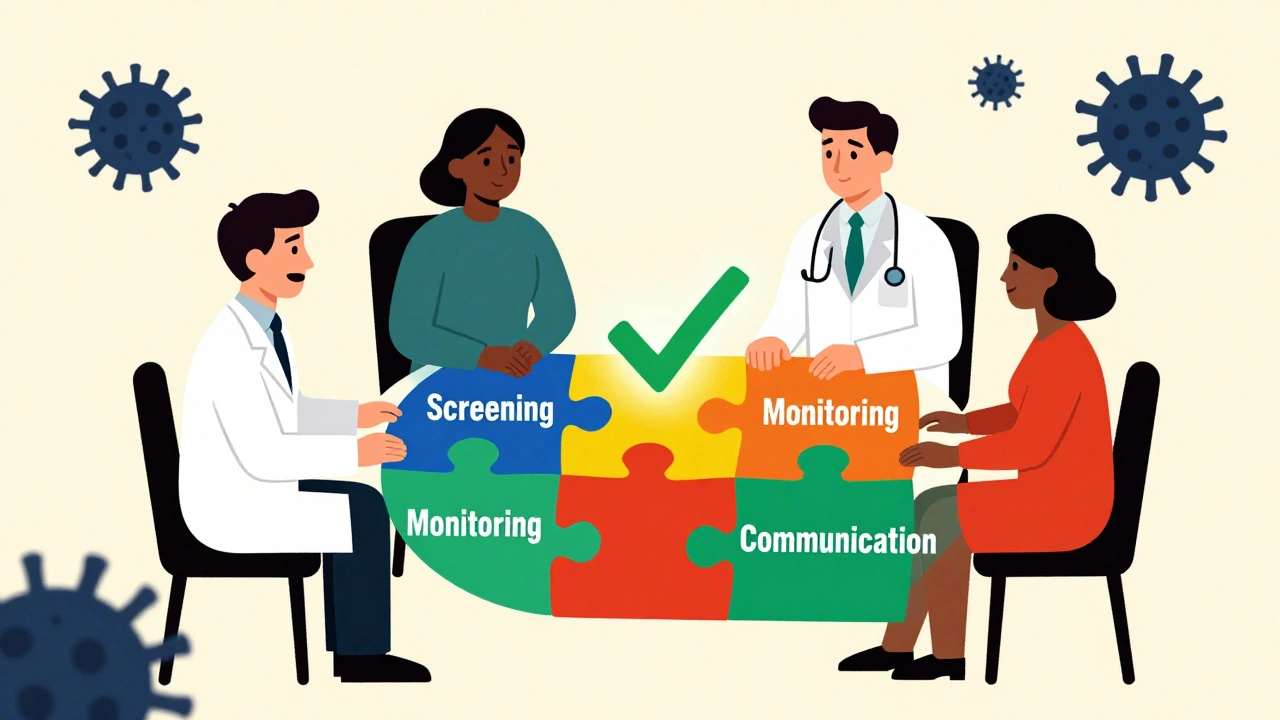
How Is Recurrence Monitored Now?
With the fear of immunosuppressants fading, the focus has shifted to smart monitoring. You’re not left on your own. Here’s what most clinics do now:
- Regular cancer screenings based on your history: mammograms, colonoscopies, skin checks, blood tests-no changes there.
- More frequent follow-ups in the first year after restarting treatment: every 3-6 months, not yearly.
- Baseline imaging if your cancer was advanced (Stage II or higher): a CT or PET scan before restarting therapy, then repeat in 6-12 months.
- Tracking symptoms: unexplained weight loss, new lumps, persistent fatigue, or night sweats get investigated fast.
- Communication between your oncologist and your rheumatologist or gastroenterologist: no more silos.
It’s not about avoiding treatment. It’s about managing it carefully. Think of it like driving a car after a crash: you don’t stop driving. You check the brakes, get a tune-up, and pay attention to the road.
What If You’re Still Worried?
It’s okay to be nervous. Cancer changes how you see your body. If you’ve been told you can’t take your medication because of your history, ask for the data. Bring up the 2016 and 2024 studies. Ask: “Is this based on evidence, or just old habit?”
Some doctors still hold onto the old rules. But the science is clear. If your autoimmune disease is active and your cancer has been in remission for at least a year, immunosuppressants are likely safe. The bigger risk isn’t cancer coming back-it’s letting your arthritis, Crohn’s, or psoriasis run wild. Chronic inflammation increases heart disease, organ damage, and even your risk of new cancers over time.
The Bottom Line
You don’t need to wait five years. You don’t need to avoid biologics. You don’t need to suffer through pain and fatigue because of outdated fears. The data says: immunosuppressants don’t cause cancer to return in most cases. The key is personalization. Your cancer type, stage, how long you’ve been in remission, and how badly your autoimmune disease is affecting your life all matter more than arbitrary time limits.
If you’re on immunosuppressants and had cancer in the past, talk to your care team. Get a clear plan: what screenings you need, how often, and who’s responsible for tracking them. Don’t let fear make you choose between pain and risk. You can have both control over your autoimmune disease and peace of mind about your cancer.”
Do immunosuppressants increase the risk of cancer coming back?
No, large, high-quality studies involving over 24,000 patients show no increased risk of cancer recurrence with anti-TNF drugs, methotrexate, azathioprine, JAK inhibitors, or newer biologics like ustekinumab. The fear that immunosuppressants cause cancer to return is not supported by current evidence.
Should I wait five years after cancer to start immunosuppressants?
No. The five-year rule was based on guesswork, not science. Studies show no difference in recurrence whether you start treatment six months or six years after cancer diagnosis. Treatment decisions should be based on your cancer type, stage, remission status, and how severe your autoimmune disease is-not an arbitrary timeline.
Are some cancers riskier than others when taking immunosuppressants?
Yes. Most cancers-like breast, colon, and prostate-show no increased recurrence risk. But melanoma and active blood cancers (like leukemia or lymphoma) may be different. Immune surveillance plays a bigger role in controlling these cancers, so doctors often recommend longer remission periods or extra monitoring. Always discuss your specific cancer type with your care team.
Is it safe to use combination therapy if I’ve had cancer?
The data doesn’t show that combination therapy (like anti-TNF + methotrexate) increases recurrence risk compared to single drugs. However, combination therapy had the highest numerical recurrence rate in studies, so doctors may be more cautious with it in high-risk cases. The decision should be individualized, not automatic.
What kind of cancer monitoring is needed after restarting immunosuppressants?
You should continue your regular cancer screenings (mammograms, colonoscopies, skin checks). In the first year after restarting treatment, many clinics recommend more frequent check-ins (every 3-6 months) and possibly a baseline imaging scan if your cancer was advanced. Report any new symptoms-weight loss, lumps, night sweats-right away. Coordination between your oncologist and rheumatologist is key.
Have official guidelines changed because of this research?
Yes. The FDA updated drug labels in 2022 to reflect that clinical studies show no increased cancer recurrence risk. The American College of Rheumatology and EULAR now recommend individualized risk assessments instead of blanket waiting periods. Treatment should be based on your cancer’s type, stage, and remission duration-not a fixed number of years.
Comments (11)
Raja Herbal
December 9, 2025 AT 15:20
So let me get this straight-we spent 20 years scaring people out of life-saving meds because some doctor read a textbook from 1998? And now we’re just… admitting it was nonsense? Classic.
Rich Paul
December 9, 2025 AT 16:08
bro the anti-TNFs are literally just chillin’ in your system like a bae who doesn’t ghost u, and the immune system’s like ‘oh hey u still here? cool’ and just keeps doin’ its job. no magic wand, no cancer gremlins, just science. also methotrexate is still kinda sus but not *that* sus. 🤷♂️
Ruth Witte
December 9, 2025 AT 18:59
YAS THIS IS THE INFO WE NEEDED!! 🙌 No more living in fear of your own meds!! You deserve to feel good without guilt!! Go talk to your doc and demand a plan!! You got this!! 💪❤️
Katherine Rodgers
December 11, 2025 AT 11:05
ohhh so now it’s ‘the data says’? funny how the same people who screamed ‘pharma lies!’ about vaccines are suddenly quoting JAMA like it’s gospel. i’ll believe it when i see 10-year data. until then, i’m keepin’ my meds on ice.
Lauren Dare
December 12, 2025 AT 02:24
Let’s be real: the 5-year rule was never about evidence. It was about liability. Doctors didn’t want to be the one who said ‘go ahead’ and then had a recurrence. So they hid behind tradition. Now that the data’s here, the liability’s still there. Hence the ‘individualized risk assessment’-aka, ‘I’m still scared, but I’ll say it’s fine if you sign this waiver.’
Gilbert Lacasandile
December 13, 2025 AT 20:37
That makes a lot of sense. I’ve been on adalimumab for 3 years since my colon cancer remission-3 years ago. My oncologist and rheumatologist talk every month. I get a CT every 6 months. It’s not perfect, but it feels manageable. I’m glad the guidelines are catching up.
Lola Bchoudi
December 13, 2025 AT 23:51
For anyone reading this and feeling overwhelmed: you’re not alone. The shift from fear to informed action is huge. Start by printing out the 2024 study summary. Bring it to your next appointment. Ask: ‘Based on my cancer type and remission timeline, what’s my personalized monitoring plan?’ You’re not asking for permission-you’re claiming agency. That’s power.
Morgan Tait
December 14, 2025 AT 10:14
Did you know the FDA’s 2022 update was pushed by Big Pharma lobbyists who own half the hospitals? And the ‘24,000 patient study’? Totally funded by AbbVie. The real risk isn’t cancer recurrence-it’s the silent erosion of your immune system by synthetic biologics that haven’t been tested beyond 5 years. You think they’d let you take this if it was dangerous? They’d make you pay $12,000/month for it. 🤔
Nikhil Pattni
December 14, 2025 AT 21:44
bro i had melanoma in 2020 and started adalimumab in 2022, just 2 years after surgery. my derm said 'if it comes back, we catch it early.' and guess what? i got my 2-year skin check last week-clean as a whistle. also i use emojis now bc why not 😎🩺
Courtney Black
December 15, 2025 AT 17:34
It’s funny how we treat medicine like a moral test. You’re either ‘brave’ for taking the drug or ‘cautious’ for avoiding it. But the truth? It’s not about courage. It’s about statistics. If your risk of recurrence is 8% without treatment and 9% with it, that’s not a gamble-it’s a rounding error. The real villain is chronic inflammation, which quietly turns your body into a tinderbox for new cancers, heart attacks, and dementia. We’ve been fighting the wrong ghost. The enemy isn’t the drug. It’s the silence. The fear. The outdated dogma masquerading as care. We don’t need more rules. We need more data-and the courage to follow it, even when it contradicts everything we were taught.
Delaine Kiara
December 15, 2025 AT 18:23
So… I’m just gonna say this out loud: I’ve been on JAK inhibitors for 18 months since my breast cancer remission. My mom cried when I told her. My sister said I was ‘playing Russian roulette with my DNA.’ But I’ve had zero side effects, my psoriasis is gone, and I just ran a 5K. I’m not ‘lucky.’ I’m informed. And if you’re scared? That’s okay. But don’t let fear make you miss your life.